Neurology
Browse to: Trigeminal Neuralgia | Focal Epilepsy
Trigeminal Neuralgia
Trigeminal Neuralgia is a devastating disorder characterized by frequent, debilitating, and recurrent paroxysmal pain attacks. Trigeminal Neuralgia affects the trigeminal nerve, the fifth cranial nerve that innervates the face, causing severe, “electric shock-like” facial pain.
Trigeminal Neuralgia affects approximately 400,000 people in the United States and European Union.
Current treatments include anticonvulsants, which can have significant tolerability issues and waning efficacy over time. Medically-refractory patients may be candidates for neurosurgical interventions, which are invasive and carry significant risks.
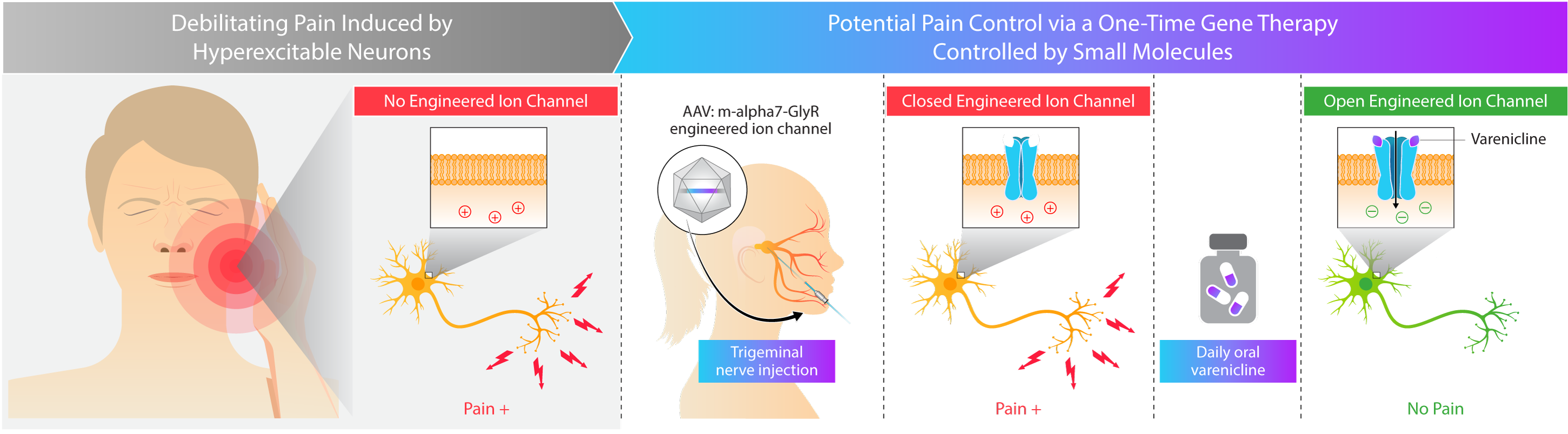
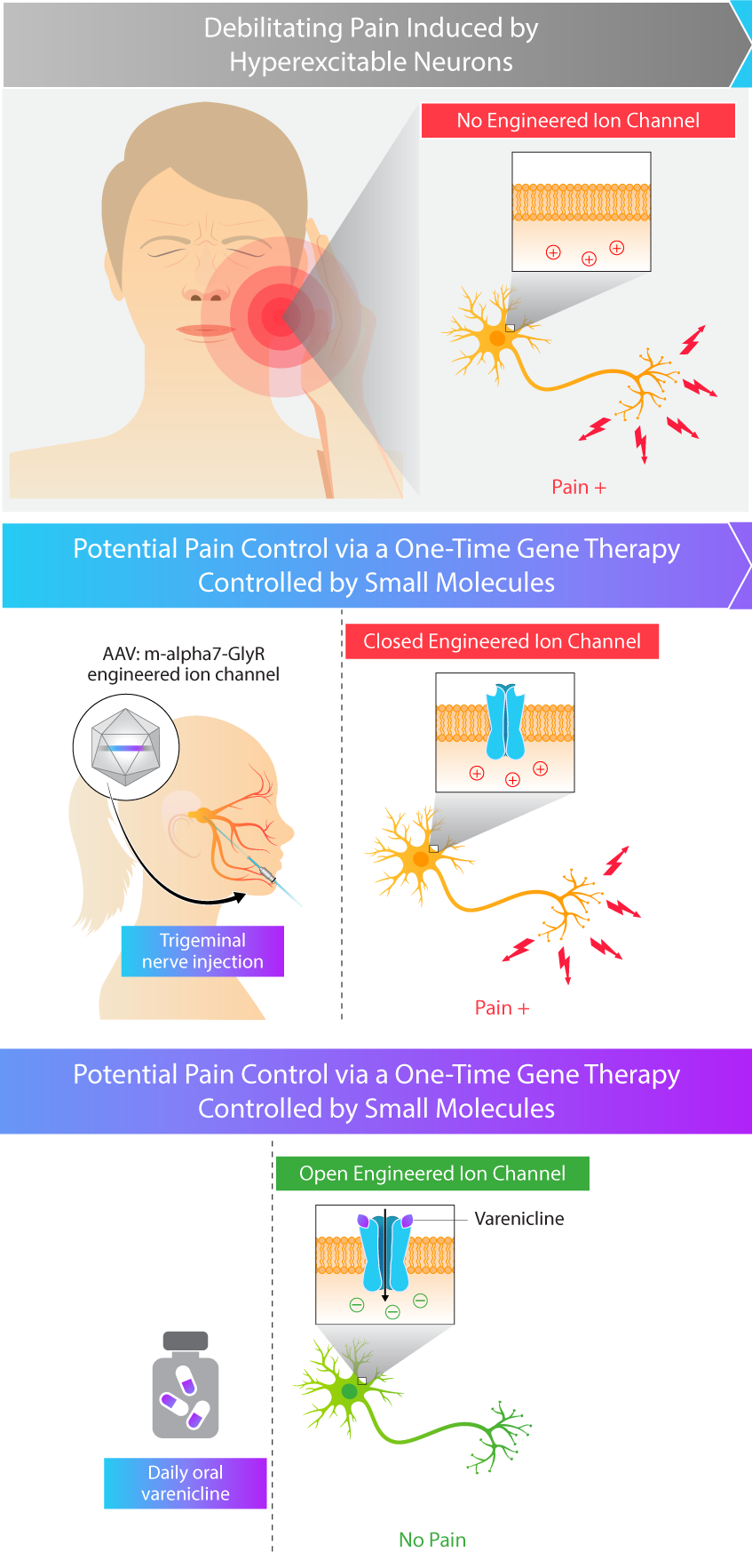
Kriya is developing KRIYA‑748, a potential one-time gene therapy for trigeminal neuralgia that expresses a chemogenetically-gated ion channel and is designed to be administered as an injection into the trigeminal nerve, with the objective of reducing the frequency and severity of pain attacks. Once expressed in the nerve, this channel is designed to selectively open in the presence of varenicline, an orally-administered small molecule that penetrates the central nervous system (CNS), leading to the passage of chloride ions and reduced excitation of target neurons. Varenicline is an FDA-approved generic medication for smoking cessation, originally marketed under the brand name “Chantix®”.
Kriya has designed KRIYA‑748 for trigeminal neuralgia with the following potential goals in mind:
- Pain reduction. Reducing the frequency and severity of pain attacks by focally inhibiting neuronal hyperexcitability in the trigeminal nerve
- Long-term durability. One-time administration of gene therapy to eliminate the need for cycling through anti-epileptic medications and surgical procedures
- Regulatable activity. Targeted delivery to the trigeminal nerve, additionally engineered with the ability to “turn off” the gene therapy through withdrawal of the orally-administered small molecule, varenicline
Approach to Treating Trigeminal Neuralgia with Gene Therapy
There is strong experimental evidence that neurons and associated behavioral networks are shaped by the interactions of ion channels expressed in specific neuronal groups. Kriya’s adeno-associated virus (AAV) gene therapy is designed to durably express an engineered ion channel focally within the trigeminal nerve, which may provide targeted pain relief. Preclinical studies have demonstrated that this ion channel is selectively opened in the presence of varenicline, an orally-administered and CNS-penetrant small molecule, leading to the passage of chloride ions and reduced excitation of target neurons. Kriya believes that this approach to treating trigeminal neuralgia may provide targeted and durable pain control – compared to anticonvulsants that may wane in efficacy over time or invasive neurosurgical interventions that carry procedural risks.
Focal Epilepsy
Focal Epilepsy is a neurological condition characterized by recurrent and spontaneous seizures that originate from a localized brain region.
Focal epilepsy affects close to 5 million people in the United States and European Union.
Current treatments include first and second line antiseizure medications. While these medications may be effective for a period of time, in some cases they do not provide adequate seizure control. Even with existing treatments, a significant proportion of patients suffer from uncontrolled, intractable, or drug-resistant seizures, a condition known as refractory epilepsy. Many of these patients are candidates for neurosurgery to remove the epileptic foci responsible for triggering seizures. However, this type of surgery is invasive and carries significant risks associated with resection of brain tissue. As a result, only a small fraction of surgically-eligible epilepsy patients undergo surgery each year.
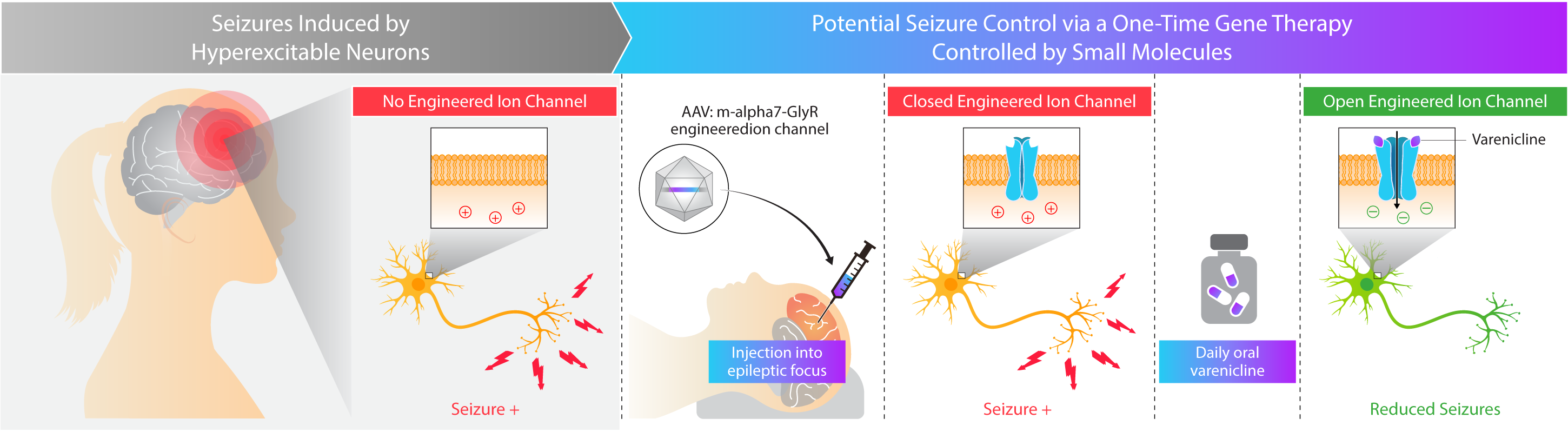
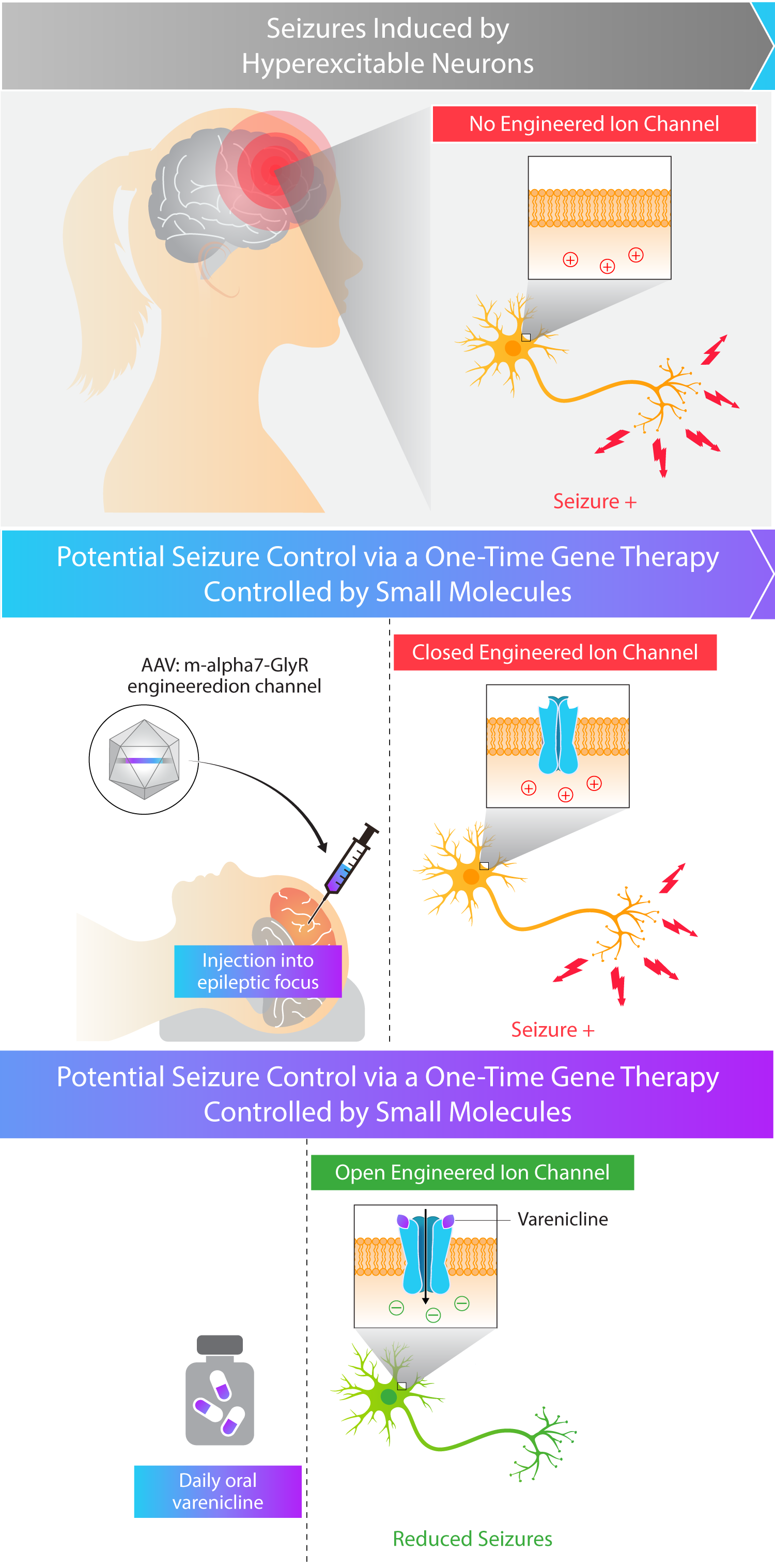
Kriya is developing KRIYA‑382, a potential one-time gene therapy for focal epilepsy that expresses a chemogenetically-gated ion channel and is designed to be administered through direct injection into epileptic foci within the brain, with the objective of reducing the frequency and severity of seizures. Once expressed in the epileptic foci, this channel is designed to selectively open in the presence of varenicline, an orally-administered small molecule that penetrates the central nervous system (CNS), leading to the passage of chloride ions and reduced excitation of target neurons. Varenicline is an FDA-approved generic medication for smoking cessation, originally marketed under the brand name “Chantix®”.
Kriya has designed KRIYA‑382 for focal epilepsy with the following potential goals in mind:
- Seizure reduction. Minimizing seizures by inhibiting neuronal hyperexcitability at epileptic foci
- Long-term durability. One-time administration of gene therapy designed to eliminate the need for cycling through anti-epileptic medications
- Regulatable activity. Targeted delivery to epileptic foci, additionally engineered with the ability to “turn off” the gene therapy through withdrawal of the orally-administered small molecule, varenicline

Approach to Treating Focal Epilepsy with Gene Therapy
Kriya’s AAV gene therapy is designed to express an engineered ion channel locally within epileptic foci to potentially reduce the burden of seizures in patients. This ion channel is designed to be selectively opened in the presence of varenicline, an orally-administered and CNS-penetrant small molecule, leading to the passage of chloride ions and reduced excitation of target neurons. Kriya hopes to demonstrate that this approach to treating focal epilepsy may provide seizure control with multi-year durability, potentially eliminating or delaying the need for more invasive neurosurgical procedures that involve the removal of sections of the brain, which carries the risk of hemorrhage, stroke, and cognitive impairment.