Research & Development

Research & Development
Historically, innovation in gene therapy has often been driven by research groups with limited in-house capabilities for optimizing and scaling product candidates, leading to challenges when attempting to translate new discoveries into successful clinical studies and commercial product launches. Kriya is pioneering a new paradigm for gene therapy R&D that empowers our team of gene therapy veterans to overcome these challenges and operate at the scale needed to address common diseases.
At each stage of the product life-cycle, we are intentional about laying a solid foundation for future commercialization and leveraging our unique in-house capabilities in computational biology and large-scale GMP manufacturing. In doing so, we have built a powerful gene therapy engine that can recurrently generate potentially transformational genetic medicines for diseases affecting millions of patients worldwide.
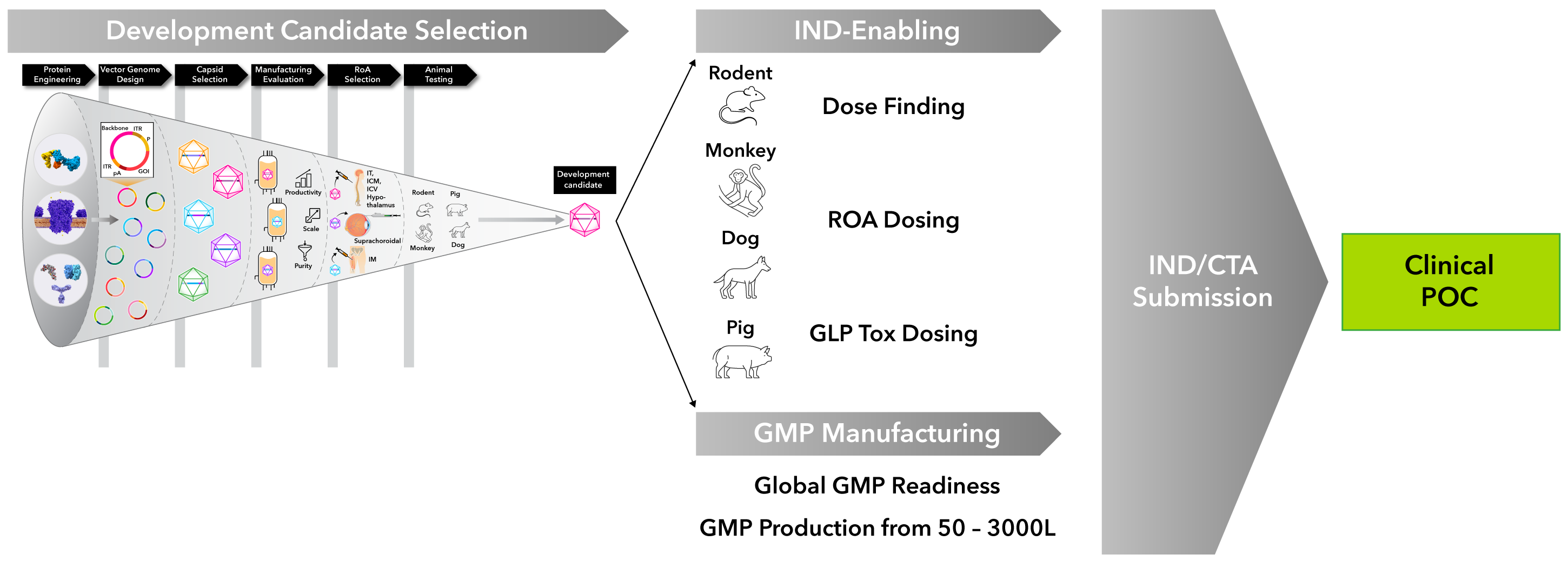
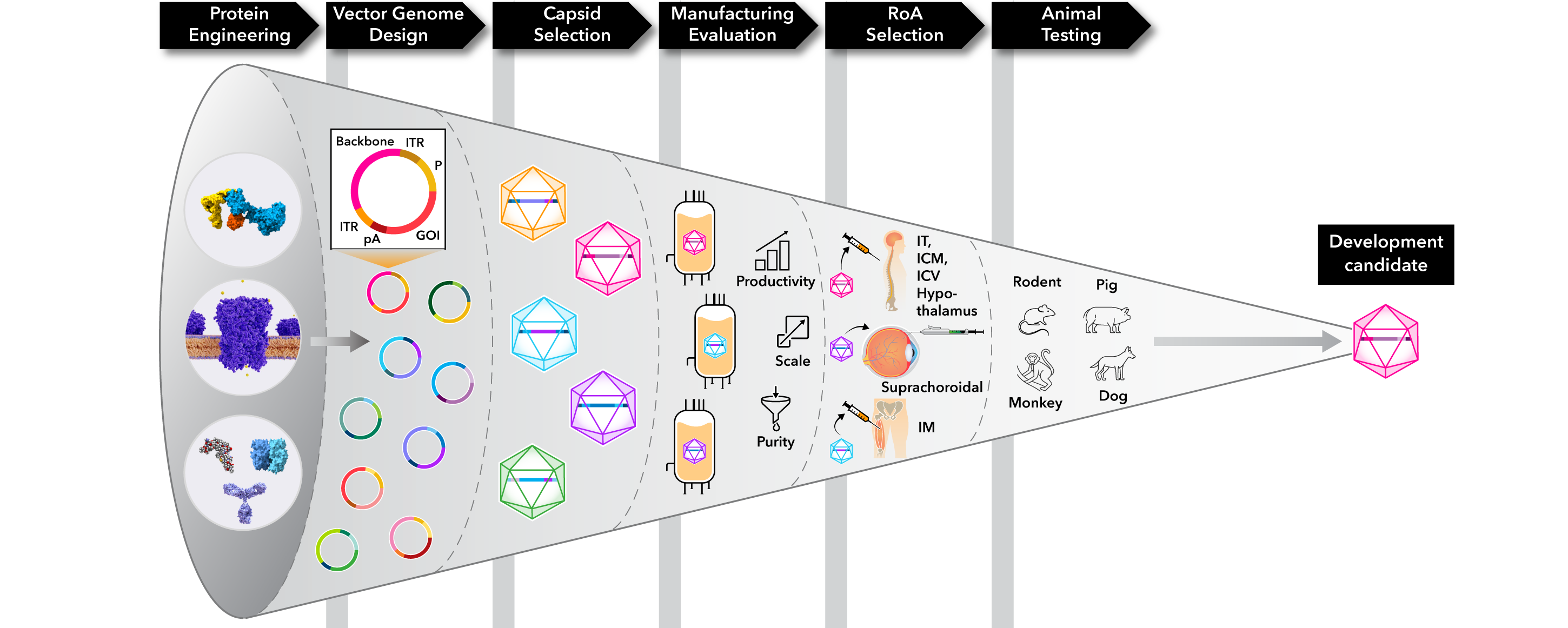
Our R&D engine includes the following steps:
- Protein Engineering: Enhance manufacturability and biological activity through computationally-driven design
- Vector Genome Design: Optimize expression, tissue specificity, manufacturability, and stability by combining cutting-edge machine learning tools with human expertise
- Capsid Selection: Select the appropriate capsid from known, clinically-validated serotypes to optimize transduction and expression in target tissues
- Manufacturing Evaluation: Assess and optimize for manufacturability in early development to ensure that final development candidates can be manufactured at scale, while avoiding delays and added costs from process changes later in development
- Route of Administration (RoA) Selection: Select RoA with the goal of maximizing therapeutic benefit and minimizing side effects by transducing target tissues with focal administration at low doses
- Animal Testing: Conduct data-driven selection of lead candidates using representative animal models
Once development candidates have been selected, we conduct animal studies that lay the foundation for regulatory submission (IND/CTA) and clinical trials. Our in-house, large-scale GMP manufacturing capabilities enable us to manufacture our own investigational products for both preclinical and clinical studies, helping to ensure consistency and high product quality.